Medical / Pharmaceutical
Hanmi Begins U.S. Trials for 'Dream Obesity Drug'
Dong-A Ilbo |
Updated 2025.11.06
U.S. FDA Approves Hanmi's HM17321 Phase 1 IND
Achieves Simultaneous Weight Loss and Muscle Gain… "Improves Exercise and Metabolic Functions"
Fundamentally Differentiated from Existing GLP-1 Therapies
Potential for Combination Therapy, Anticipated to Enhance Quantitative and Qualitative Obesity Treatment
Achieves Simultaneous Weight Loss and Muscle Gain… "Improves Exercise and Metabolic Functions"
Fundamentally Differentiated from Existing GLP-1 Therapies
Potential for Combination Therapy, Anticipated to Enhance Quantitative and Qualitative Obesity Treatment
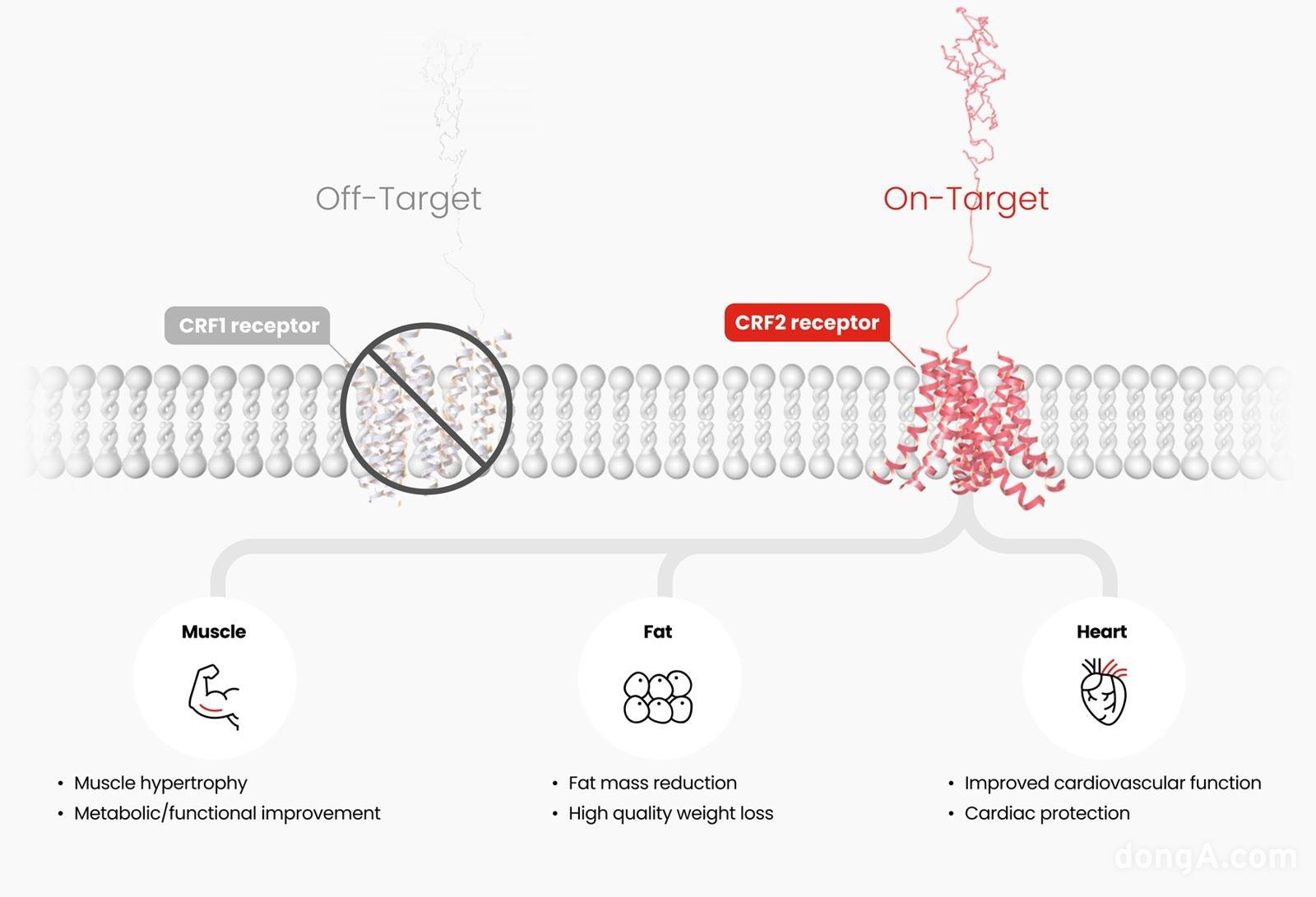
Hanmi Pharmaceutical's HM17321 selectively acts only on the CRF2 receptor (On-Target) to simultaneously induce muscle increase and fat reduction. This is expected to result in muscle enhancement, metabolic and functional improvement, quality weight loss, and cardiovascular improvement.
Hanmi Pharmaceutical is set to commence clinical trials in the United States for a 'dream obesity drug' that reduces weight while increasing muscle mass. Existing glucagon-like peptide-1 (GLP-1) based drugs, which focus on weight reduction, are known to inevitably cause muscle loss, but Hanmi Pharmaceutical has taken a step closer to developing an innovative new obesity drug that overcomes this issue.Hanmi Pharmaceutical announced on the 6th that it has received approval from the U.S. Food and Drug Administration (FDA) for the Investigational New Drug (IND) application to enter Phase 1 clinical trials for the obesity treatment 'HM17321 (LA-UCN2)'.
This Phase 1 trial will focus on evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of HM17321 in healthy adults and obese patients.
According to Hanmi Pharmaceutical, HM17321 goes beyond merely compensating for muscle loss and is characterized by achieving what was previously considered impossible: simultaneous muscle mass increase and selective fat reduction. It is being developed as a 'First-in-Class' innovative obesity drug.
Designed as a UCN2 (Urocortin-2) analogue that selectively targets the CRF2 (corticotropin-releasing factor 2) receptor rather than incretin receptors such as GLP-1, it utilizes advanced artificial intelligence (AI) and structural modeling technology embedded in the Hanmi Pharmaceutical R&D Center. CRF is known as a signaling molecule related to stress response and recovery. Hanmi Pharmaceutical explains that selectively targeting the CRF2 receptor can directly lead to fat reduction, muscle increase, and improvement in muscle function.
Particularly, it is reported to present a new paradigm for obesity treatment as a monotherapy, while also expecting quantitative and qualitative weight loss synergy in combination therapy with existing incretin-based obesity treatments. It is evaluated to have secured innovative scalability in terms of future applicability.
Hanmi Pharmaceutical stated, “Most antibody-based muscle preservation drugs have reduced convenience for obese patients due to intravenous administration, and limitations are pointed out due to differences in administration methods when combined with existing subcutaneous obesity treatments,” adding, “Side effects and mere muscle mass preservation with limited efficacy in functional muscle improvement are also cited as drawbacks.”
In contrast, “HM17321 is designed as a peptide-based substance, enhancing administration convenience and is expected to be competitive in terms of cost,” and “if developed as a combination therapy, it can be administered at once with existing incretin-based drugs in the same peptide form, further improving patient convenience,” it was reported.
Overview of Hanmi Pharmaceutical's Obesity New Drug H.O.P Project
Hanmi Obesity New Drug Project 'H.O.P' Progress
Hanmi Pharmaceutical has announced the 'H.O.P (Hanmi Obesity Pipeline)' obesity new drug project and is developing a total of six pipelines. The first product of the H.O.P project is 'Efeglenatide'. Previously, it announced the interim topline results of Phase 3 clinical trials, predicting a leap as a 'national obesity drug' based on excellent efficacy and safety.The second pipeline, a next-generation obesity treatment triple agonist (HM15275, LA-GLP/GIP/GCG), submitted an IND for Phase 2 clinical entry to the U.S. FDA in July and received approval. It is being developed as a 'Best-in-Class' new drug with commercialization targeted for 2030.
The third pipeline, HM17321, has further increased the likelihood of clinical success based on preclinical and mechanistic research results using overweight and obese primate models. This treatment aims for commercialization by 2031.
Choi In-young, Executive Director of Hanmi Pharmaceutical R&D Center, stated, “HM17321 is not merely a weight loss drug but aims for simultaneous fat reduction, muscle increase, and improvement in exercise and metabolic functions, fundamentally differentiating it from existing treatments,” adding, “Hanmi Pharmaceutical approaches obesity not as a competition of numbers on a scale but as a resolution of the fundamental causes of metabolic diseases, providing 'patient-centered customized solutions' and opening new horizons in obesity treatment.”
Kim Min-beom
AI-translated with ChatGPT. Provided as is; original Korean text prevails.
ⓒ dongA.com. All rights reserved. Reproduction, redistribution, or use for AI training prohibited.
Popular News