Medical / Pharmaceutical
Nasal Spray Flu Vaccine Introduced for Children
Dong-A Ilbo |
Updated 2025.10.15
Broader Options for Flu Vaccination
Type A and B Mainly Infect Humans… Vaccines 'Updated' According to Virus
Nasal Spray Vaccine Introduced This Year… Effective for Children with Cellular Immune Response
Type A and B Mainly Infect Humans… Vaccines 'Updated' According to Virus
Nasal Spray Vaccine Introduced This Year… Effective for Children with Cellular Immune Response
According to the World Health Organization (WHO), seasonal flu infects approximately 1 billion people worldwide each year, with 3 million to 5 million cases resulting in severe illness. Respiratory-related deaths due to flu reach 290,000 to 650,000 annually.
Why Annual Vaccination is Necessary
‘Virus Mutation’ and ‘Decreased Immunity’
Influenza is categorized into types A, B, and C, with types A and B primarily causing infections in humans. It spreads through droplets expelled by coughing or sneezing of infected individuals, and the contagious period can be longer for children or those with weakened immunity.
Infection results in sudden onset of systemic symptoms such as high fever, chills, headache, muscle aches, and fatigue, along with respiratory symptoms like cough, sore throat, and runny nose. The symptoms are severe and rapid, making daily activities difficult and potentially leading to complications such as pneumonia, necessitating caution.
Due to the constant mutation of the flu virus, the prevalent strains change annually. Additionally, immunity naturally decreases over time. Therefore, seasonal flu vaccines are updated each year to match the circulating virus, and annual vaccination is necessary for adequate prevention.
Flu vaccination is the most effective method to prevent flu and reduce the risk of severe complications and death. Vaccination is particularly important for children, who are at higher risk of infection. An analysis of 32 clinical trials showed seasonal flu incidence rates of 12.7% in children, 4.4% in adults, and 7.2% in those aged 65 and older.
Shin Kwang-chul, director of Mirae Otolaryngology Clinic, emphasized, “Children are more vulnerable to flu and more likely to spread the virus after infection, making vaccination crucial. Annual vaccination is necessary to build immunity against the circulating virus.”
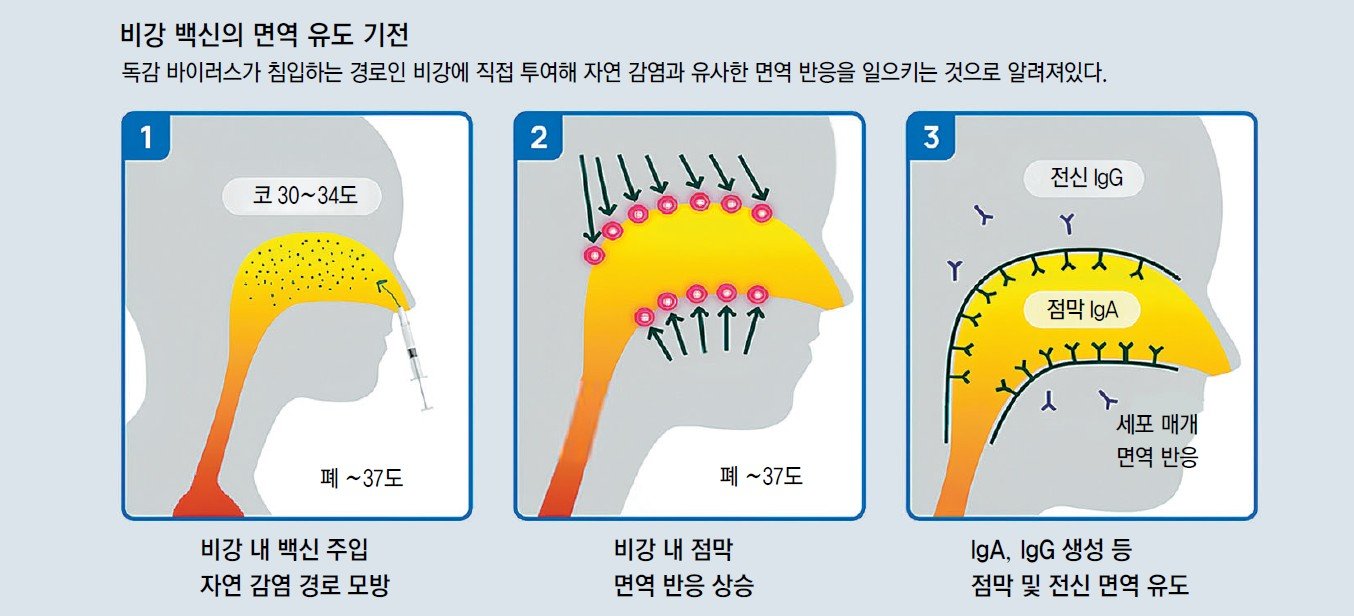
Nasal Spray Vaccine Available This Year
Although the mechanism of immune induction by this vaccine is not fully understood, it is administered directly to the nasal cavity, the natural route of flu virus entry, eliciting an immune response similar to natural infection. This activates both mucosal and cellular immune responses in the respiratory tract, which is advantageous.
FluMist is administered by spraying once into each nostril without injection pain. It has shown relatively high preventive efficacy in children. Even when the antigen does not match, it provides higher preventive efficacy than the injectable inactivated vaccine, which may be advantageous for annually changing virus strains.
Additional analysis of the MI-CP111 clinical trial conducted during the 2004-2005 season showed that in children aged 24 to 59 months, the live vaccine group had a 52.5% (95% CI 26.7-69.7) reduction in flu incidence for antigen-matched viruses and a 54.2% (95% CI 38.8-66.0) reduction for antigen-mismatched viruses compared to the inactivated vaccine group. Director Shin noted, “Nasal spray vaccines have been introduced domestically in the past and can be considered as an alternative option for children with a fear of injections.”
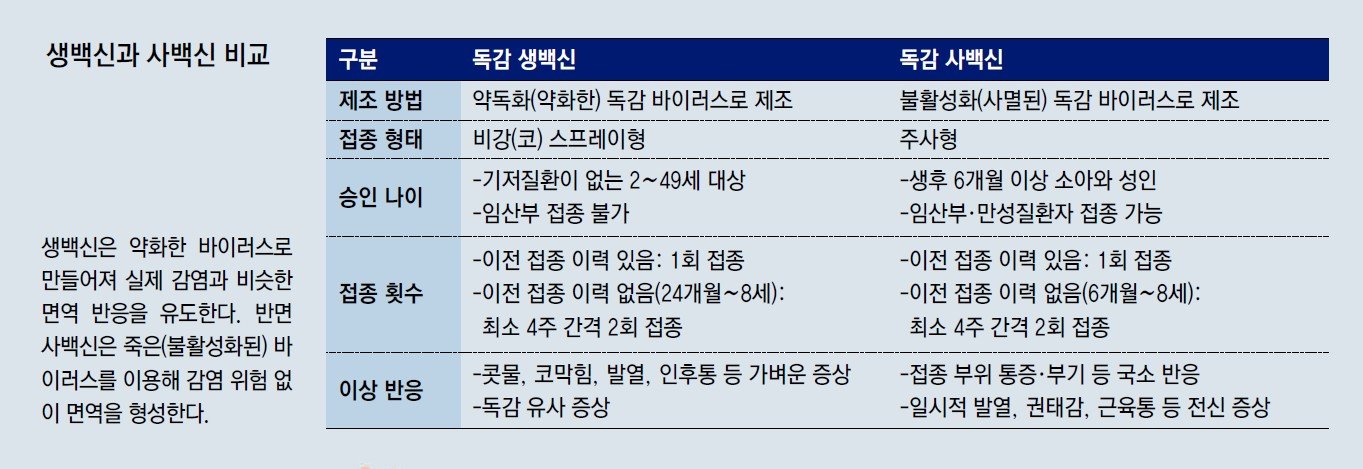
Understanding the Pros and Cons of Live and Inactivated Vaccines
The attenuated live vaccine is made from weakened viruses and is administered as a nasal spray. It is intended for individuals aged 2 to 49 without underlying health conditions. Children aged 24 months to 8 years who have not previously received a flu vaccine require two doses four weeks apart, while those with prior vaccination history or aged 9 to 49 need only one dose.
In contrast, the inactivated vaccine is an injectable vaccine made by inactivating the virus with heat or chemicals. It can be used for all age groups from 6 months and older, including pregnant women and those with chronic conditions. Children aged 6 months to 8 years with no prior vaccination history require two doses four weeks apart, while those with previous vaccination experience or aged 9 and older need only one dose.
Hong Eun-sim
AI-translated with ChatGPT. Provided as is; original Korean text prevails.
ⓒ dongA.com. All rights reserved. Reproduction, redistribution, or use for AI training prohibited.
Popular News