Previously 420 days… Longer than the US and Japan, slowing new drug introduction and undermining competitiveness
MFDS has pursued streamlined procedures since last year… Drug approvals up 68% versus 2024
This year to introduce preliminary review and parallel assessment… 198 additional specialists to be hired
In November last year, the adult epilepsy treatment “Excopri Tablet” (ingredient name: cenobamate) obtained marketing authorization from the Ministry of Food and Drug Safety (MFDS) 256 days after filing the application. The usual marketing authorization period for a new drug had been around 420 days, meaning the process was shortened by more than five months. This is the first case in which the MFDS applied the “New Drug Marketing Authorization and Review Procedures” guideline introduced to accelerate new drug approvals. Patients who had been inconvenienced by having to obtain prescriptions for this drug overseas due to the absence of a domestic treatment option will now see their burden eased.
Going forward, the review period for new drug approvals is expected to become even shorter. The MFDS has set a goal of gradually reducing the authorization period for medical products such as biopharmaceuticals to an average of 240 days this year. To achieve this, it plans to improve authorization procedures and significantly expand its review workforce.
● “Delays in authorization reviews are weakening bio competitiveness”The MFDS’s authorization and review process is designed to verify whether medical products can be used safely and effectively by the public. Based on data submitted by companies, it scientifically evaluates the product’s quality, safety, and efficacy. Although this is an essential procedure, there have been persistent criticisms that it has taken excessively long for new medical products to be launched after obtaining MFDS approval. On average, the authorization period for new drugs has been 420 days, for biosimilars (follow-on biopharmaceuticals) 406 days, and for new medical devices 398 days.
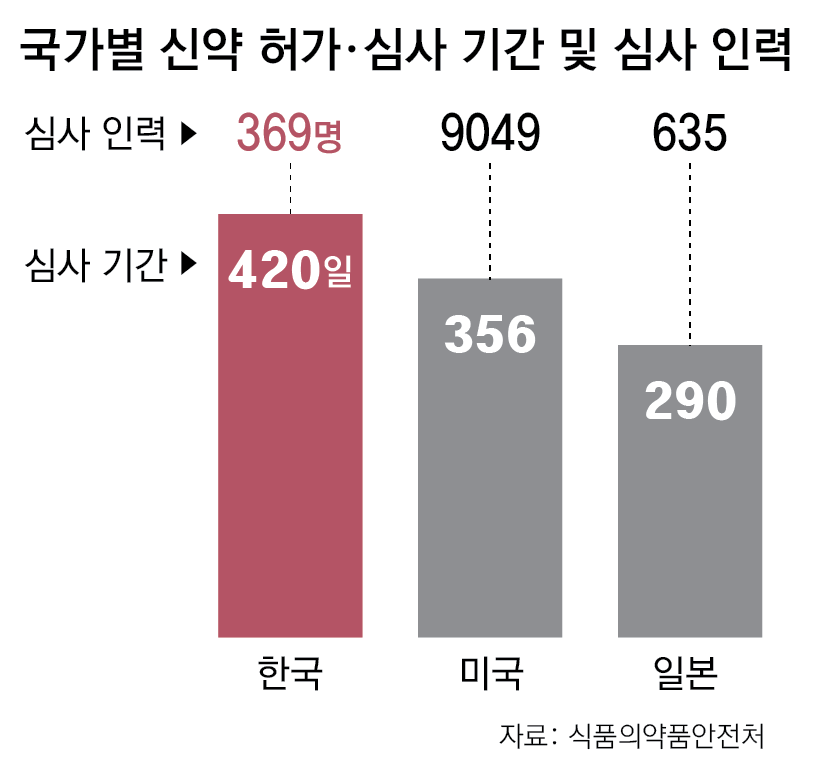
By contrast, the average new drug review period at the U.S. Food and Drug Administration (FDA) is 356 days, and at Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) it is 290 days, roughly four months faster than in Korea. As a result, there has been ongoing criticism that outdated review procedures are slowing the introduction of new drugs and undermining the competitiveness of Korea’s bioindustry.
In response, the government announced the “K-Bio Pharmaceutical Industry Grand Leap Strategy” in September last year, stating that it would “shorten authorization review periods by four months to drive growth of the K-bio pharmaceutical industry in the global market.” At an MFDS work briefing in December last year, President Lee Jae-myung directly instructed MFDS Commissioner Oh Yu-kyung to “pursue measures to reduce the review period to the shortest possible timeframe, on the premise of safety,” adding that “personnel expansion to support this must proceed in parallel.”
● New drug review period to be shortened from 420 days to 240 daysThe MFDS has presented a target of reducing the average authorization period for new drugs and similar products to 240 days this year. Since last year, it has improved its review procedures by introducing face-to-face reviews in place of the previous written Q&A format, and by specifying that the Good Manufacturing Practice (GMP) review period must be within 90 days.
As a result, the number of drug approvals last year totaled 405, an increase of 20.9% compared to 335 in 2024. In particular, in the second half of the year (July–December), after the launch of the new administration, 225 approvals were granted, up 25% from 180 in the first half (January–June). Compared to the same period in 2024 (134 cases), this represents a 67.9% surge.
This year as well, the MFDS plans to accelerate reviews, led by the Biopharmaceutical Products Division, by introducing in-depth preliminary reviews and concurrent and parallel reviews by review item. The U.S. FDA operates a preliminary review system before the formal start of the review to prevent delays caused by missing data or errors in documentation. Parallel review deploys specialized reviewers for each area, such as quality and clinical results, simultaneously to save time.
● 198 authorization and review staff to be hired this yearTo shorten authorization review periods, the MFDS also plans to increase its staffing. The current number of MFDS personnel dedicated to medical product authorization and review is 369. By comparison, the U.S. FDA has 9,049 reviewers, the European Medicines Agency (EMA) has about 4,000, and in Japan 635 reviewers were employed as of last year. While the number of medical product authorizations in Korea is equivalent to 80–90% of those in the U.S. and Europe, staffing amounts to only 4% of that of the U.S. and 9% of that of Europe. The MFDS estimates that approximately 300 additional staff members are needed to reach the level of Japan.
The MFDS has decided to hire 198 new staff this year, equivalent to about 50% of its existing authorization and review personnel. This will be the largest expansion of staff since the MFDS was established. By field, it will recruit 19 general civil servants responsible for pharmacy and medical technology, 177 research officers in public health and industrial research, and 2 general fixed-term civil servants. They will be tasked with formulating policies related to medical product authorization and conducting safety and efficacy reviews of medical products. Although the MFDS headquarters and the National Institute of Food and Drug Safety Evaluation, which is in charge of reviews, are located in Osong, Cheongju, there are six regional offices in major metropolitan cities such as Seoul, Busan, and Daejeon, enabling rotational assignments.
An MFDS official stated, “We will help newly hired staff develop expertise by providing specialized training in review fields,” adding, “We look forward to creating a future in which Korean new drugs lead overseas markets together with talented professionals.”
The application period runs until the 20th of this month. Detailed information on eligibility requirements and application methods can be found on the MFDS Excellent Talent Recruitment System (employ.mfds.go.kr).
ⓒ dongA.com. All rights reserved. Reproduction, redistribution, or use for AI training prohibited.
Popular News
