Biopharma
Targeted Cancer Radiopharmaceuticals Intensify Global Crown Race
Dong-A Ilbo |
Updated 2026.02.10
[Young Old&] Minimizing side effects by limiting damage to healthy cells
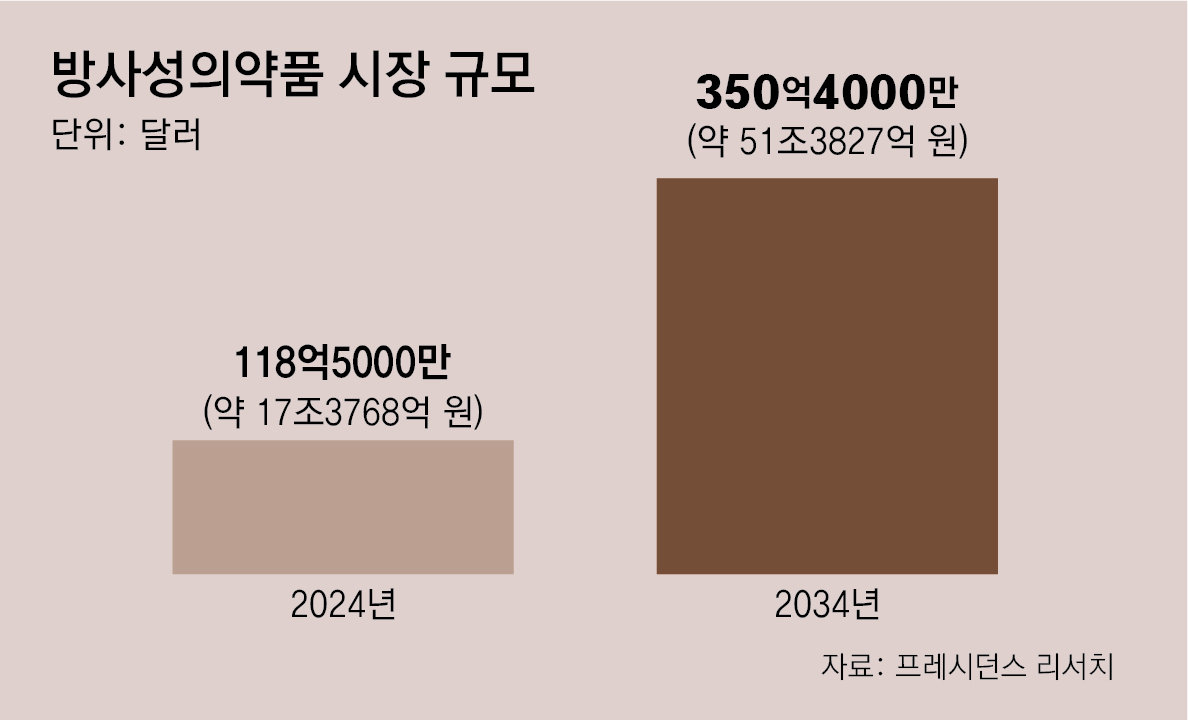
Global market projected at KRW 51 trillion by 2034
Novartis’ Pluvicto sales surge as drugmakers race to invest in production facilities
SK Biopharm also launches full-scale push
Global market projected at KRW 51 trillion by 2034
Novartis’ Pluvicto sales surge as drugmakers race to invest in production facilities
SK Biopharm also launches full-scale push
According to the pharmaceutical and biotech industry on the 9th, global pharmaceutical companies that have been actively pursuing mergers and acquisitions (M&A) of radiopharmaceutical developers over the past two to three years are now moving to build out supply chains. Novartis, a frontrunner in the radiopharmaceuticals market, announced earlier this year that it would establish a fourth dedicated radiopharmaceutical manufacturing facility in Winter Park, Florida, in the United States. Through this, the company aims to stabilize its radiopharmaceutical supply chain and maintain its dominant position in the global market.
● Market projected at over KRW 50 trillion by 2034
Prostate cancer radiopharmaceutical ‘Pluvicto’. Courtesy of Novartis
The potential of radiopharmaceuticals was demonstrated by Novartis’ prostate cancer therapy Pluvicto. Approved by the U.S. Food and Drug Administration (FDA) in 2022, Pluvicto generated USD 1.994 billion (approximately KRW 2,924 billion) in sales last year, up 42% from the previous year. However, unlike other pharmaceuticals, radioactive materials are difficult to produce and handle, and their half-life (the time required for radioactivity to decrease by half) is short, making the establishment of production bases a major challenge.View of Novartis’ radiopharmaceutical manufacturing facility in Carlsbad, California, United States
Vas Narasimhan, Chief Executive Officer (CEO) of Novartis, who is preparing for the construction of the company’s fourth dedicated manufacturing facility, stated, “By building new radiopharmaceutical plants, we will be able to supply medicines to patients more quickly and reliably,” adding, “If the Winter Park plant starts operating in 2029 as planned, we will be able to maintain a radiopharmaceutical dosing rate of over 99%.”Latecomers are also resolving supply chain risks by acquiring biotech companies that already have radiopharmaceutical production facilities. Bristol Myers Squibb (BMS) acquired U.S. biotech company RayzeBio in 2024 for USD 4.1 billion (approximately KRW 6,012.2 billion) and is operating a production facility in Indiana, United States. AstraZeneca likewise acquired Fusion Pharmaceuticals, which has manufacturing facilities, for USD 2.4 billion (approximately KRW 3,519.4 billion).
● SK Biopharmaceuticals takes the lead in Korea
In Korea, SK Biopharmaceuticals, which has built its reputation as a next-generation “new drug powerhouse” by developing an innovative epilepsy treatment, has entered the radiopharmaceuticals field. This is also a key business led under the full authority of Choi Yoon-jung, Head of Strategy at SK Biopharmaceuticals and eldest daughter of SK Group Chairman Chey Tae-won.
SK Biopharmaceuticals is also moving to establish production bases in multiple locations, including the United States, Germany, and Belgium. In 2024, the company introduced its first radiopharmaceutical drug candidate, SKL35501, from Hong Kong-based Full-Life Technologies, and on 12 January this year received FDA approval for a Phase 1 clinical trial (IND). While developing the drug, the company has also signed supply agreements with global radiopharmaceutical raw material companies such as TerraPower in the United States, PanTera in Belgium, and Eckert & Ziegler in Germany, thereby securing regional production hubs. In his New Year’s address this year, SK Biopharmaceuticals CEO Lee Dong-hoon stated, “Radiopharmaceuticals are a market where there is still no clearly defined global leader,” and expressed his determination by adding, “We will ensure that our first-mover opportunity translates into tangible results.”
Choi Ji-won
AI-translated with ChatGPT. Provided as is; original Korean text prevails.
ⓒ dongA.com. All rights reserved. Reproduction, redistribution, or use for AI training prohibited.
Popular News