Startup / Biotech / FDA
Medirule: Overcoming FDA Hurdles with AI SaaS
Dong-A Ilbo |
Updated 2025.12.08
[SeoulTech x IT Donga Joint Planning] Seoul National University of Science and Technology (hereafter referred to as SeoulTech) operates various support programs to assist the growth of startups, including preliminary and early-stage startup packages, maker spaces, and global collaborations. Furthermore, in collaboration with IT Donga, it provides global news to aid startups in overseas promotion and expansion, introducing promising deep-tech startups both domestically and internationally.
The FDA (U.S. Food and Drug Administration) is an agency under the U.S. Department of Health and Human Services that oversees the safe and effective distribution of food, pharmaceuticals, medical devices, and cosmetics in the United States. In Korea, it is commonly known through marketing phrases like ‘FDA certification,’ but strictly speaking, it is a regulatory agency, not a certification body. To aid understanding regarding sales permits and approvals related to the FDA, it is referred to as ‘certification.’
It is naturally challenging to pass the FDA's threshold. According to Emergo by UL, a company related to medical device regulations, it is estimated that of the approximately 15,100 submissions to the FDA CDRH (Center for Devices and Radiological Health) in 2024, only 5,564 were approved. In 2023, 19,100 submissions were made, with 5,807 approvals, and in 2022, 5,731 approvals were granted. Even without considering the number of approvals by specific category, only about 30% of all submissions can pass the FDA's threshold.
“AI-implemented devices have a higher recall probability... Active management based on AI needed”
Ahn Mi-kyung, with extensive experience related to the FDA and medical device planning, has a career spanning approximately 20 years. Ahn stated, “Having worked as a planner in the medical device field for nearly 20 years and managing regulations, I obtained the ISO 13485 (an international standard for quality management systems in the medical device industry) certification. I mainly evaluated development suitability while handling FDA certifications for medical devices in the practical field. I created medical services in collaboration with Korea University Anam Hospital and developed a telemedicine platform with Catholic University Medical School.”
The reason for establishing Medirule is due to the winds of change blowing across the FDA market because of AI. “While planning, I confirmed that medical devices incorporating recent artificial intelligence or machine learning tend to have more errors, and I thought this issue would increase if applied digitally,” she said. In 2021, Ahn investigated the recall possibility of AI-based medical devices through the study ‘Research dynamics in medical errors and design types using text mining of FDA medical device recall data,’ and many studies have shown this to be true.
Medical devices with AI show a peculiar pattern in recall rates compared to the total number of devices applied. According to a 2025 study by the Journal of the American Medical Association (JAMA), AI devices developed by public companies account for only 53% of all devices but represent 92% of recalls. AI device recalls are concentrated within 1 to 2 years, with errors mainly due to software, data processing, and algorithm performance degradation. Devices lacking clinical verification data are found to have a higher recall risk. As AI medical devices increase, the recall risk rises, necessitating a mediator to manage risk factors and support companies as the FDA expands digital applications.
FDA transitions 510(k), PMA, De Novo to electronic systems from October this year
Meanwhile, starting October 1 this year, the FDA's expansion of online applications and electronic submission systems for medical devices is expected to bring significant changes to application and approval rates. Since October 2023, the FDA has mandated 510(k) submissions to be made via eSTAR electronic submissions, with De Novo also being mandated in the same manner from October 2025. This means submissions can no longer be made via paper or email, and only the eSTAR electronic format is recognized. On the other hand, PMA remains a voluntary choice, with paper submissions still possible.
510(k) applies when there is an equivalent device on the market, like a blood glucose meter, PMA is for high-risk devices requiring clinical trials, like pacemakers, and De Novo applies when there is no existing similar device on the market and the risk is low. De Novo includes technologies like AI imaging diagnostic software and app-based health management systems.
With the ability to apply digitally for PMA and De Novo, doors have opened for small and medium-sized enterprises and startups, but the reality remains challenging. Ahn Mi-kyung stated, “The proportion of startups among FDA applicant companies is currently very low,” adding, “With the FDA opening the door to online applications, the situation can rapidly advance from now on. Medirule aims to assist companies with FDA support and reduce their burden through regulatory management methods via AI SaaS.”
21-step process in total... In Korea, supported by government projects
How does one obtain FDA approval? Ahn Mi-kyung explained, “If there is a similar medical device, apply with 510(k), and if it is a life-critical device, apply as a PMA device with clinical trials attached. For new technology categories without equivalent devices on the market, you can directly request classification through the De Novo route, and there is also the Humanitarian Device Exemption (HDE) for devices targeting limited patient populations. Once the application route is determined, necessary materials such as test results or conformity tests must be uploaded step by step in the eSTAR program. The FDA's review targets are 90 days for 510(k), 150 days for De Novo, and 180 days for PMA, which is significantly shortened from the several months it used to take.”
In Korea, it is common to apply through government support projects such as dedicated personnel, overseas licensing support projects, overseas standard certification acquisition support projects, and export vouchers. In contrast, in the United States, the FDA certification process is industrialized. Enzyme is servicing AI SaaS focused on FDA regulatory automation, and companies like Greenlight Guru and MasterControl Solutions focus on quality management systems for medical devices. Companies like NAMSA have teams called ‘US FDA Consulting’ that design FDA licensing on behalf of others.
Both domestically and internationally, large companies employ dedicated FDA response personnel as a basic practice, and medium-sized companies also prioritize FDA personnel highly. Conversely, startups and small and medium-sized enterprises, which are burdened by manpower and funds, find it challenging to pass the FDA's threshold. Even if they manage to obtain FDA approval, systematizing it for long-term management is not easy. However, with the advent of digital applications, alternatives must emerge for startups and small and medium-sized enterprises to apply for FDA easily and systematically, and Medirule aims to capture this market.
SaaS optimized for rapid change, FDA certification, and management
Ahn Mi-kyung has built up to the minimum viable product (MVP) of Medirule AI SaaS and plans to start the service in earnest next year. What are the advantages that can be gained through AI SaaS? Ahn stated, “Most people involved, such as FDA certification staff and dedicated consultants, say it would be good to speed up the certification process. If entrusted to government projects or external agencies, there are delays as materials go back and forth, and security issues arise. Also, if there are multiple certified devices, management becomes difficult.”
She continued, “Managing in the form of SaaS makes it easy to protect data with the latest security policies and manage documents by project. Materials can be managed regardless of internal or external relations. In fact, the most time-consuming part of the FDA certification process is document supplementation, and if this is done within the service, the speed will definitely increase.” Additionally, as records accumulate for each company, it is advantageous in terms of handover or data protection even if the person in charge changes in the future.
The company can use SaaS directly, but Medirule also directly handles the FDA registration process. Ahn Mi-kyung stated, “Based on FDA registration know-how, we also assist in the FDA registration of companies using the service. In this case, the company using the service only needs to manage the documents without having to employ dedicated FDA personnel, saving both manpower and time. Regarding costs, we plan to set it at a level that feels reasonable in the market, and we intend to keep it lower than Enzyme.”
“Service background prepared with support from SeoulTech... Business to be concretized from next year”
Ahn Mi-kyung, while developing Medirule AI SaaS, also publishes a newsletter containing FDA trends every week. Ahn stated, “According to the medical device industry report, 85% of companies receiving FDA approval say regulatory compliance is difficult. It is harder to comply with regulations than to raise funds for approval. To respond quickly to the market, I personally check the FDA announcements daily and process them into a newsletter format. From next year, it will be serviced for a fee, and readers say it helps with quick regulatory responses.” With the FDA's workforce reduction and eSTAR expansion this year, it is recommended for those involved in medical device development and regulation to keep up with industry news through the newsletter.
Next year, through corporate support projects, the business will be put on track, and the MVP form will be advanced to introduce formal services, as well as find ways to directly link with eSTAR within the service. Ahn Mi-kyung stated, “The Medirule service can be utilized not only in Korea but also by companies in the U.S. or other countries outside the U.S. Additionally, for service convenience, we will research ways to apply directly to the FDA.”
Ahn Mi-kyung expressed gratitude to the SeoulTech Startup Support Group for enabling focus on the business. Ahn stated, “Currently in the preliminary startup stage, we are working behind the scenes for corporate registration next year. In this process, we have applied for the preliminary startup package from the SeoulTech Startup Support Group and received help. Although there were many difficulties as it was my first startup, I received comprehensive support such as legal mentoring, operational management suggestions, promotion, and funding.”
She continued, “I also participated in networking days with global expansion companies, where I shared ways to overcome difficulties with individual entrepreneurs in similar situations and found clues on what problems to solve. Next year, I aim to lay the foundation for becoming an AI SaaS company sought after by medical device manufacturers worldwide.”
IT Donga Nam Si-hyun Reporter (sh@itdonga.com)
The FDA (U.S. Food and Drug Administration) is an agency under the U.S. Department of Health and Human Services that oversees the safe and effective distribution of food, pharmaceuticals, medical devices, and cosmetics in the United States. In Korea, it is commonly known through marketing phrases like ‘FDA certification,’ but strictly speaking, it is a regulatory agency, not a certification body. To aid understanding regarding sales permits and approvals related to the FDA, it is referred to as ‘certification.’
Ahn Mi-kyung, CEO of Medirule, has over 20 years of experience in medical device planning and big data construction / Source=IT Donga
It is naturally challenging to pass the FDA's threshold. According to Emergo by UL, a company related to medical device regulations, it is estimated that of the approximately 15,100 submissions to the FDA CDRH (Center for Devices and Radiological Health) in 2024, only 5,564 were approved. In 2023, 19,100 submissions were made, with 5,807 approvals, and in 2022, 5,731 approvals were granted. Even without considering the number of approvals by specific category, only about 30% of all submissions can pass the FDA's threshold.
“AI-implemented devices have a higher recall probability... Active management based on AI needed”
Ahn Mi-kyung, with extensive experience related to the FDA and medical device planning, has a career spanning approximately 20 years. Ahn stated, “Having worked as a planner in the medical device field for nearly 20 years and managing regulations, I obtained the ISO 13485 (an international standard for quality management systems in the medical device industry) certification. I mainly evaluated development suitability while handling FDA certifications for medical devices in the practical field. I created medical services in collaboration with Korea University Anam Hospital and developed a telemedicine platform with Catholic University Medical School.”
Ahn Mi-kyung has published a paper on the increase in medical device errors due to AI. As such cases increase, changes are required in the FDA approval-related industry / Source=IT Donga
The reason for establishing Medirule is due to the winds of change blowing across the FDA market because of AI. “While planning, I confirmed that medical devices incorporating recent artificial intelligence or machine learning tend to have more errors, and I thought this issue would increase if applied digitally,” she said. In 2021, Ahn investigated the recall possibility of AI-based medical devices through the study ‘Research dynamics in medical errors and design types using text mining of FDA medical device recall data,’ and many studies have shown this to be true.
Medical devices with AI show a peculiar pattern in recall rates compared to the total number of devices applied. According to a 2025 study by the Journal of the American Medical Association (JAMA), AI devices developed by public companies account for only 53% of all devices but represent 92% of recalls. AI device recalls are concentrated within 1 to 2 years, with errors mainly due to software, data processing, and algorithm performance degradation. Devices lacking clinical verification data are found to have a higher recall risk. As AI medical devices increase, the recall risk rises, necessitating a mediator to manage risk factors and support companies as the FDA expands digital applications.
FDA transitions 510(k), PMA, De Novo to electronic systems from October this year
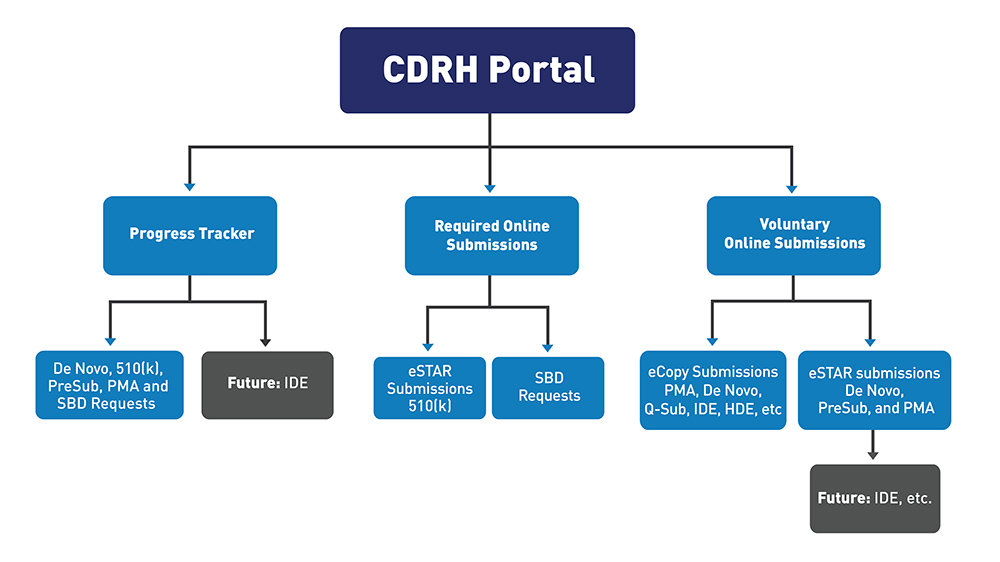
Meanwhile, starting October 1 this year, the FDA's expansion of online applications and electronic submission systems for medical devices is expected to bring significant changes to application and approval rates. Since October 2023, the FDA has mandated 510(k) submissions to be made via eSTAR electronic submissions, with De Novo also being mandated in the same manner from October 2025. This means submissions can no longer be made via paper or email, and only the eSTAR electronic format is recognized. On the other hand, PMA remains a voluntary choice, with paper submissions still possible.
Structure of the FDA's medical device review document submission and management portal. PMA and De Novo have recently changed to mandatory eSTAR submissions, and the scope of PDF-based electronic submissions has expanded / Source=FDA
510(k) applies when there is an equivalent device on the market, like a blood glucose meter, PMA is for high-risk devices requiring clinical trials, like pacemakers, and De Novo applies when there is no existing similar device on the market and the risk is low. De Novo includes technologies like AI imaging diagnostic software and app-based health management systems.
With the ability to apply digitally for PMA and De Novo, doors have opened for small and medium-sized enterprises and startups, but the reality remains challenging. Ahn Mi-kyung stated, “The proportion of startups among FDA applicant companies is currently very low,” adding, “With the FDA opening the door to online applications, the situation can rapidly advance from now on. Medirule aims to assist companies with FDA support and reduce their burden through regulatory management methods via AI SaaS.”
21-step process in total... In Korea, supported by government projects
How does one obtain FDA approval? Ahn Mi-kyung explained, “If there is a similar medical device, apply with 510(k), and if it is a life-critical device, apply as a PMA device with clinical trials attached. For new technology categories without equivalent devices on the market, you can directly request classification through the De Novo route, and there is also the Humanitarian Device Exemption (HDE) for devices targeting limited patient populations. Once the application route is determined, necessary materials such as test results or conformity tests must be uploaded step by step in the eSTAR program. The FDA's review targets are 90 days for 510(k), 150 days for De Novo, and 180 days for PMA, which is significantly shortened from the several months it used to take.”
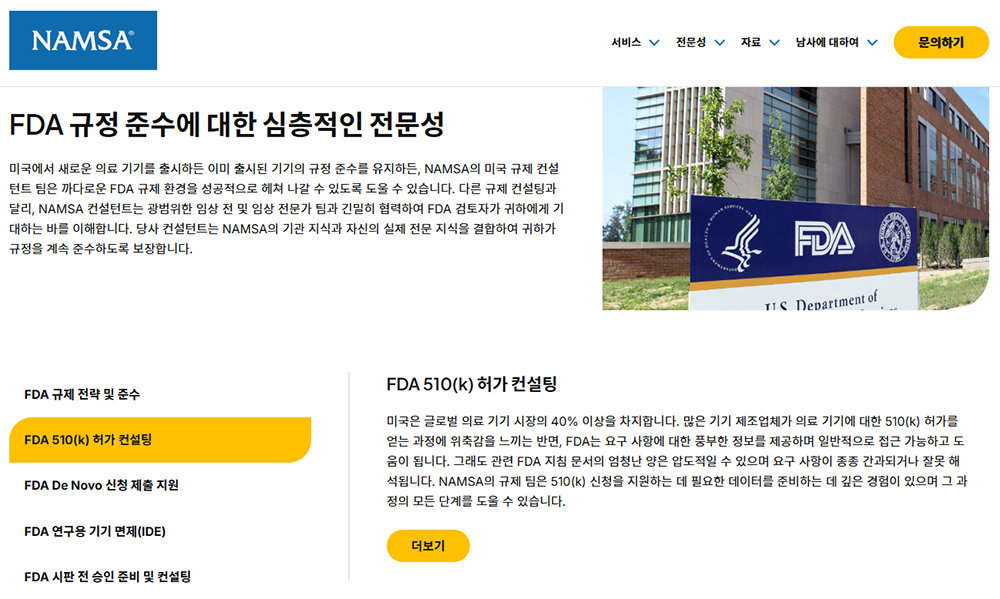
Many companies, like NAMSA, operate specialized consulting teams for FDA approval / Source=NAMSA
In Korea, it is common to apply through government support projects such as dedicated personnel, overseas licensing support projects, overseas standard certification acquisition support projects, and export vouchers. In contrast, in the United States, the FDA certification process is industrialized. Enzyme is servicing AI SaaS focused on FDA regulatory automation, and companies like Greenlight Guru and MasterControl Solutions focus on quality management systems for medical devices. Companies like NAMSA have teams called ‘US FDA Consulting’ that design FDA licensing on behalf of others.
Both domestically and internationally, large companies employ dedicated FDA response personnel as a basic practice, and medium-sized companies also prioritize FDA personnel highly. Conversely, startups and small and medium-sized enterprises, which are burdened by manpower and funds, find it challenging to pass the FDA's threshold. Even if they manage to obtain FDA approval, systematizing it for long-term management is not easy. However, with the advent of digital applications, alternatives must emerge for startups and small and medium-sized enterprises to apply for FDA easily and systematically, and Medirule aims to capture this market.
SaaS optimized for rapid change, FDA certification, and management
Ahn Mi-kyung has built up to the minimum viable product (MVP) of Medirule AI SaaS and plans to start the service in earnest next year. What are the advantages that can be gained through AI SaaS? Ahn stated, “Most people involved, such as FDA certification staff and dedicated consultants, say it would be good to speed up the certification process. If entrusted to government projects or external agencies, there are delays as materials go back and forth, and security issues arise. Also, if there are multiple certified devices, management becomes difficult.”
She continued, “Managing in the form of SaaS makes it easy to protect data with the latest security policies and manage documents by project. Materials can be managed regardless of internal or external relations. In fact, the most time-consuming part of the FDA certification process is document supplementation, and if this is done within the service, the speed will definitely increase.” Additionally, as records accumulate for each company, it is advantageous in terms of handover or data protection even if the person in charge changes in the future.
The company can use SaaS directly, but Medirule also directly handles the FDA registration process. Ahn Mi-kyung stated, “Based on FDA registration know-how, we also assist in the FDA registration of companies using the service. In this case, the company using the service only needs to manage the documents without having to employ dedicated FDA personnel, saving both manpower and time. Regarding costs, we plan to set it at a level that feels reasonable in the market, and we intend to keep it lower than Enzyme.”
“Service background prepared with support from SeoulTech... Business to be concretized from next year”
Ahn Mi-kyung, while developing Medirule AI SaaS, also publishes a newsletter containing FDA trends every week. Ahn stated, “According to the medical device industry report, 85% of companies receiving FDA approval say regulatory compliance is difficult. It is harder to comply with regulations than to raise funds for approval. To respond quickly to the market, I personally check the FDA announcements daily and process them into a newsletter format. From next year, it will be serviced for a fee, and readers say it helps with quick regulatory responses.” With the FDA's workforce reduction and eSTAR expansion this year, it is recommended for those involved in medical device development and regulation to keep up with industry news through the newsletter.
A part of the newsletter published by Ahn Mi-kyung after direct research, it will be converted to a paid subscription from next year / Source=IT Donga
Next year, through corporate support projects, the business will be put on track, and the MVP form will be advanced to introduce formal services, as well as find ways to directly link with eSTAR within the service. Ahn Mi-kyung stated, “The Medirule service can be utilized not only in Korea but also by companies in the U.S. or other countries outside the U.S. Additionally, for service convenience, we will research ways to apply directly to the FDA.”
Ahn Mi-kyung expressed gratitude to the SeoulTech Startup Support Group for enabling focus on the business. Ahn stated, “Currently in the preliminary startup stage, we are working behind the scenes for corporate registration next year. In this process, we have applied for the preliminary startup package from the SeoulTech Startup Support Group and received help. Although there were many difficulties as it was my first startup, I received comprehensive support such as legal mentoring, operational management suggestions, promotion, and funding.”
She continued, “I also participated in networking days with global expansion companies, where I shared ways to overcome difficulties with individual entrepreneurs in similar situations and found clues on what problems to solve. Next year, I aim to lay the foundation for becoming an AI SaaS company sought after by medical device manufacturers worldwide.”
IT Donga Nam Si-hyun Reporter (sh@itdonga.com)
AI-translated with ChatGPT. Provided as is; original Korean text prevails.
ⓒ dongA.com. All rights reserved. Reproduction, redistribution, or use for AI training prohibited.
Popular News